Synthesis of quinolines and naphthyridines via catalytic retro-aldol reaction of β-hydroxyketones with ortho-aminobenzaldehydes or nicotinaldehydes†
Abstract
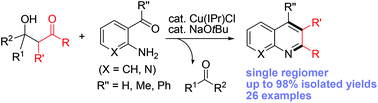
A Cu(I)-catalyzed retro-aldol reaction of β-hydroxyketones with ortho-aminobenzaldehydes and nicotinaldehydes is reported that produces a range of quinolines and naphthyridines with high efficiency and selectivity. This reaction uses β-hydroxyketones as a regiospecific ketone-protected enolate source via copper-catalyzed retro-aldol Cα–Cβ bond cleavage. The in situ generated copper enolate undergoes kinetically favorable cyclization with ortho-amino aryl aldehydes to produce quinolines and naphthyridines in a chemo- and regioselective manner. The mild and weakly basic reaction conditions also suppress possible side reactions of benzaldehydes under strongly basic conditions, resulting in improved reaction yields.

 Please wait while we load your content...
Please wait while we load your content...