Transition-metal-free oxychlorination of alkenyl oximes: in situ generated radicals with tert-butyl nitrite†
Abstract
Oxychlorination of alkenyl oximes is harder compared to the analogous oxybromination or oxyiodination because of the difficulty associated with the formation of chlorine cations or radicals. A transition-metal-free oxychlorination of alkenyl oximes has been developed, using t-BuONO as a dual oxidant and AlCl3 as a chlorine source. This convenient and practical method has been used to construct chloroisoxazolines in moderate to good yields, whereas N-chlorosuccinimide (NCS) failed to promote this reaction.
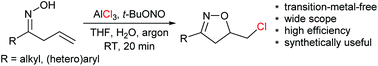

 Please wait while we load your content...
Please wait while we load your content...