Photo-cleavable nucleotides for primer free enzyme mediated DNA synthesis†
Abstract
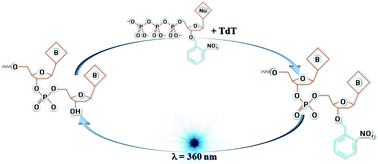
The synthesis, characterization and potential application of eight 3′-O modified 2′-deoxyribonucleoside triphosphates (dNTPs) are discussed. These nucleotide analogues are modified by capping the 3′-OH with a photolabile protecting group which can temporarily cease DNA strand growth and can smoothly reinitiate the growth by the photodecomposition of the protecting group and setting the 3′-OH of dNTPs free to propagate. The synthesis of 3′-O-(2-nitrobenzyl)-2′-deoxyribonucleoside triphosphates (NB-dNTPs) and 3′-O-(4,5-dimethoxy-2-nitrobenzyl)-2′-deoxyribonucleoside triphosphates (DMNB-dNTPs) is discussed in detail with structural confirmation using NMR. The UV-cleaving studies are monitored and quantified using LCMS and 1H NMR spectral traces. The synthesised nucleotides are employed for terminating and reinitiating template-less DNA synthesis, using primer independent Terminal Deoxynucleotidyl Transferase (TdT) enzyme. The use of this photolabile nucleotide in one step stop–start DNA synthesis is a novel strategy towards the precise assembly of dNTPs with the potential to reinforce present technologies.

 Please wait while we load your content...
Please wait while we load your content...