Temperature-dependent annuloselectivity and stereochemistry in the reactions of methanesulfonyl sulfene with imines†
Abstract
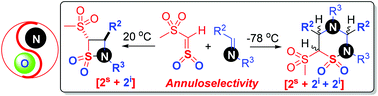
The annuloselectivity in the reactions of methanesulfonyl sulfene and imines varies with temperature. At a relatively higher temperature of 20 °C, the [2s + 2i] annulation of different N-alkyl imines occurs exclusively, giving four-membered trans-β-sultams in up to 69% yields. At a lower temperature of −78 °C, the [2s + 2i + 2i] annulation of N-methyl imines takes place specifically, delivering six-membered 1,2,4-thiadiazine 1,1-dioxides, 4-aza-δ-sultams, in up to 80% yields, with diverse configurations at the C3, C5, and C6 stereocenters. The trans-stereochemistry involved in the [2s + 2i] annulations is attributed to the conrotatory ring closure of the thermodynamically stable 2,3-thiazabutadiene-type zwitterionic intermediates, while the diverse stereochemical outcomes in the [2s + 2i + 2i] annulations are caused by the iminium isomerization in the stepwise nucleophilic [4 + 2] annulation between the same zwitterionic intermediates and a second molecule of N-methyl imines.

 Please wait while we load your content...
Please wait while we load your content...