Preparation of 3-azoindoles and 3-hydrazonoindolin-2-imines as well as their applications as NNO pincer ligands for boron†
Abstract
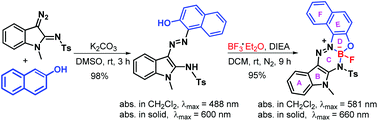
Potassium carbonate-promoted coupling reactions between 3-diazoindolin-2-imines and nucleophiles were tested. By respectively applying 2-naphthalenols and 2-arylacetates as nucleophiles, 3-azoindoles and 3-hydrazonoindolin-2-imines were obtained in excellent yields. Moreover, 3-azoindol-2-amines could be used as NNO pincer ligands for boron and resulted in the formation of hexacycleborofluorides with their absorption around 580 nm in dichloromethane.

 Please wait while we load your content...
Please wait while we load your content...