Advances in the 1-phenanthryl-tetrahydroisoquinoline series of PAK4 inhibitors: potent agents restrain tumor cell growth and invasion†
Abstract
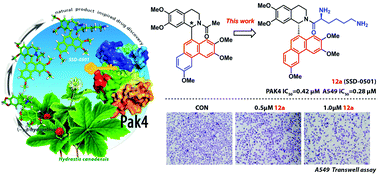
A new series of novel 1-phenanthryl-tetrahydroisoquinoline derivatives were designed, synthesized and biologically evaluated for their PAK4 inhibitory activities and anti-proliferative effects against three cancer cell lines A549, MCF-7 and HT-1080. Among them, compound 12a exhibited the most potent inhibitory activity against PAK4 with an IC50 value of 0.42 μM. Moreover, this compound inhibited the invasion of A549 tumor cells by regulating the PAK4–LIMK1–cofilin signaling pathway in vitro, and exhibited anti-tumor activity in vivo in the A549 tumor xenograft model. To further evaluate the binding mode of 12a with PAK4, the biotinylated 12a derivative has been synthesized and it was used for immunoprecipitation assay. Intriguingly, our observations suggest that 12a interacts with both the N- and C-termini of PAK4.


 Please wait while we load your content...
Please wait while we load your content...