Intramolecular hydroarylation of aryl propargyl ethers catalyzed by indium: the mechanism of the reaction and identifying the catalytic species†
Abstract
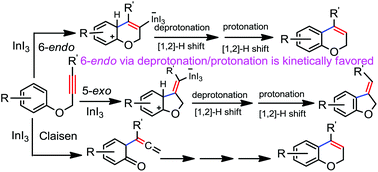
The mechanism and regioselectivity of the intramolecular hydroarylation of phenyl propargyl ether catalyzed by indium in gas and solvent phases were investigated by means of the density functional theory method. The computed results revealed that the reaction proceeds through initial π-coordination of the propargyl moiety to the catalyst, which triggers the nucleophilic attack of the phenyl ring via an exo- or endo-dig pathway in a Friedel–Crafts type mechanism. Calculation results obtained employing InI2+ as the possible catalyst show similar activation energies for the 5-exo-dig and 6-endo-dig pathways. In contrast, the neural catalyst InI3 shows a kinetic preference for 6-endo-dig versus 5-exo-dig cyclizations leading to the experimentally observed product, 2H-chromene. The calculation results suggest that InI3 could be the real catalytic species for this reaction as it shows regioselectivity in agreement with the experimental observation. Furthermore, the 6-endo-dig cyclization through deprotonation/protonation steps is kinetically more favored than the stepwise two consecutive [1,2]-H shift steps. The rate determining step of the whole catalytic cycle is the deprotonation step with an energy barrier of 18.9 kcal mol−1 in toluene solvent. The effects of substituents on both the phenyl ring and the propargyl moiety on the selectivity and elementary steps of the hydroarylation process were investigated. A methoxy group, particularly at the meta-position, on the phenyl ring largely decreases the energy barrier of the first step for the 6-endo path, though it shows little effect on the activation energies of the second and third steps. Our calculation results are in good agreement with the experimental results.

 Please wait while we load your content...
Please wait while we load your content...