Tertiary amine-catalyzed and direct synthesis of α-chloroalkanesulfonylhydrazines from azodicarboxylates and sulfonyl chlorides†
Abstract
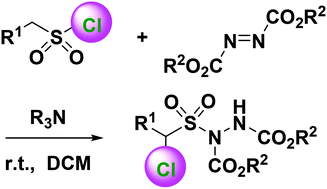
Chloroalkanesulfonylhydrazines were synthesized directly and efficiently from various alkanesulfonyl chlorides and dialkyl azodicarboxylates under the catalysis of tertiary amines. Tertiary amines serve as both bases and nucleophiles to dehydrochlorinate alkanesulfonyl chlorides to afford sulfenes. They then nucleophilically attack azodicarboxylates to yield zwitterionic intermediates, which nucleophilically attack sulfenes followed by intramolecular nucleophilic displacement and intermolecular chloride substitution to give rise to the final dialkyl α-chloroalkanesulfonylhydrazine-1,2-dicarboxylates. The proposed method provides a new and mild strategy for direct preparation of α-chloroalkanesulfonyl derivatives without other chloride resource, removing the complications incurred in traditional methods.
 Please wait while we load your content...
Please wait while we load your content...