Cobalt(iii)-catalyzed C–H halogenation of 6-arylpurines: facile entry into arylated, sulfenylated and alkoxylated 6-arylpurines†
Abstract
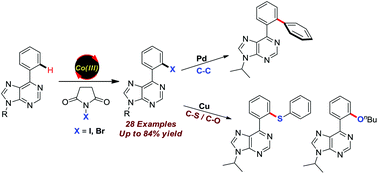
Cobalt-catalyzed C–H halogenation of biologically important 6-arylpurines has been reported under mild conditions with good functional group tolerance. The regioselective halogenation of thiophenes, as well as the synthetic applicability of the present protocol for the synthesis of arylated, sulfenylated and alkoxylated purine analogues was also demonstrated.

 Please wait while we load your content...
Please wait while we load your content...