Palladium-catalyzed β-C(sp3)–H arylation of phthaloyl alanine with hindered aryl iodides: synthesis of complex β-aryl α-amino acids†
Abstract
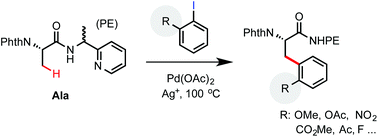
An efficient protocol for palladium-catalyzed β-C(sp3)–H arylation of aliphatic carboxamides equipped with a 2-(2-pyridyl) ethylamine (PE) auxiliary was developed. The PE auxiliary is uniquely effective at facilitating the arylation of primary C(sp3)–H bonds with sterically hindered aryl iodides. A variety of aryl iodides bearing alkoxyl, carbonyl, nitro and halogen groups on the ortho position can react with the PE-coupled phthaloyl alanine substrate in moderate to excellent yield. These reactions offer a useful solution for preparing complex β-aryl α-amino acid products from readily accessible starting materials.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...