One-pot, highly efficient, asymmetric synthesis of ring-fused piperidine derivatives bearing N,O- or N,N-acetal moieties†
Abstract
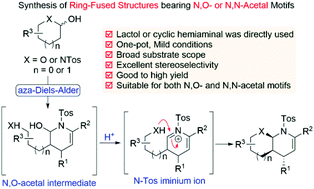
We successfully expand the application of lactols or cyclic hemiaminals as nucleophiles for the asymmetric synthesis of both N,O- and N,N-acetal moieties contained in the structure of ring-fused piperidine derivatives. This efficient one-pot protocol involves an organocatalyzed asymmetric aza-Diels–Alder reaction and iminium ion induced cyclization sequence to ultimately deliver heterocyclic compounds with excellent stereoselectivity in high yield, containing three continuous stereogenic centers.

 Please wait while we load your content...
Please wait while we load your content...