A Neber approach for the synthesis of spiro-fused 2H-azirine-pyrazolone†
Abstract
A base-mediated Neber reaction of enaminopyrazolones, which are the tautomers of 4-acyloxime-2-pyrazolin-5-ones, with sulfonyl chlorides was achieved. With this developed approach, a range of spiro-fused 2H-azirine-pyrazolones were obtained in good yields under mild conditions. A preliminary trial of a catalytic asymmetric version of the Neber reaction was conducted and gave promising enantioselectivity.
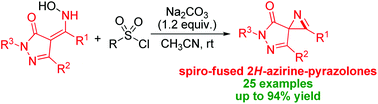

 Please wait while we load your content...
Please wait while we load your content...