Copper-catalyzed direct C–H fluoroalkenylation of heteroarenes†
Abstract
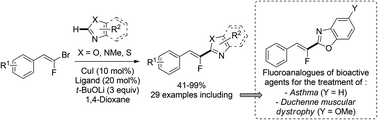
Copper-catalyzed direct C–H fluoroalkenylation of heterocycles using various gem-bromofluoroalkenes as electrophiles is reported. This efficient method offers step-economical, low-cost and stereocontrolled access to relevant heteroarylated monofluoroalkenes. The synthesis of fluorinated analogues of biomolecules and therapeutic agents for the treatment of Duchenne muscular dystrophy as application is reported.

 Please wait while we load your content...
Please wait while we load your content...