Synthesis of 3-substituted isoindolin-1-ones via a tandem desilylation, cross-coupling, hydroamidation sequence under aqueous phase-transfer conditions†
Abstract
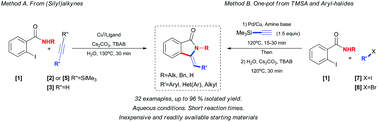
A simple and expedient method for the synthesis of 3-methylene-isoindolin-1-ones 4 under aqueous phase-transfer conditions has been developed. Starting from 2-iodobenzamides 1 and (silyl)alkynes, the products are obtained in high yields and short reaction times (30 min) with the use of inexpensive CuCl/PPh3 catalyst system in the presence of n-Bu4NBr (TBAB) as a phase-transfer agent. Terminal alkynes are conveniently “unmasked” upon in situ desilylation under the reaction conditions. Alkynes possessing heterocyclic moieties were also found as amenable substrates. Furthermore, a one-pot process starting from 2-iodobenzamides 1, aryl halides (bromides or iodides) and trimethylsilylacetylene (TMSA) as a convenient acetylene surrogate was also shown to be feasible under Pd/Cu catalysis.

 Please wait while we load your content...
Please wait while we load your content...