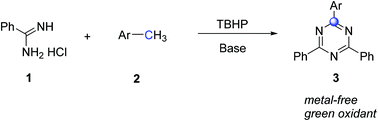
Direct oxidative coupling of amidine hydrochlorides and methylarenes: TBHP-mediated synthesis of substituted 1,3,5-triazines under metal-free conditions†
Abstract
Various 2,4,6-trisubstituted 1,3,5-triazines were smoothly formed via TBHP-mediated direct oxidative coupling of amidine and methylarenes. This tandem oxidation–imination–cyclization transformation exhibits a straightforward protocol to prepare 1,3,5-triazines from easily available starting materials and green oxidants under metal-free conditions.

 Please wait while we load your content...
Please wait while we load your content...