Total syntheses of disulphated glycosphingolipid SB1a and the related monosulphated SM1a†
Abstract
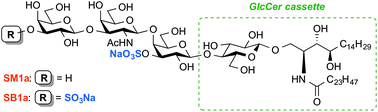
Total syntheses of two natural sulphoglycolipids, disulphated glycosphingolipid SB1a and the structurally related monosulphated SM1a, are described. They have common glycan sequences and ceramide moieties and are associated with human epithelial carcinomas. The syntheses featured efficient glycan assembly and the glucosyl ceramide cassette as a versatile building block. The binding of the synthetic sulphoglycolipids by the carcinoma-specific monoclonal antibody AE3 was investigated using carbohydrate microarray technology.

 Please wait while we load your content...
Please wait while we load your content...