Metal-free oxidative cleavage of the C–C bond in α-hydroxy-β-oxophosphonates†
Abstract
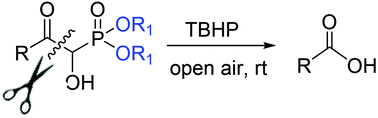
The potential of TBHP to promote oxidative hydroxylation of α-hydroxy-β-oxophosphonates (HOPs) through C(CO)–C bond cleavage is described. This cleavage, as depicted in the mechanism is expected through an isomer of HOP that reacts with TBHP to generate acids.

 Please wait while we load your content...
Please wait while we load your content...