Synthesis of fully protected, reverse N-prenylated (2S,3R)-3-hydroxytryptophan, a unique building block of the cyclomarins†
Abstract
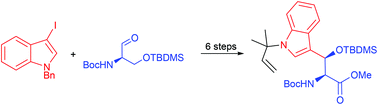
Reverse N-prenylated 3-hydroxytryptophan, the rather exotic amino acid of the cyclomarins, is obtained in enantio- and diastereomerically pure and fully protected form by a combination of a highly stereoselective addition of a zincated indole toward protected serinal and subsequent palladium-catalyzed N-prenylation.

 Please wait while we load your content...
Please wait while we load your content...