2-Oxo promoted hydrophosphonylation & aerobic intramolecular nucleophilic displacement reaction†
Abstract
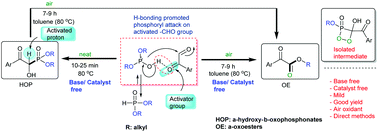
Highly efficient catalyst free methods for the synthesis of α-hydroxy-β-oxophosphonates and α-oxoesters have been described. The existence of a 2-oxo group in α-oxoaldehydes is a key factor in promoting the reaction of the tervalent phosphite form towards 2-oxoaldehydes in the synthesis of α-hydroxy-β-oxophosphonates. The in situ activated α-C–H atom of α-hydroxy-β-oxophosphonates sustains aerobic intramolecular nucleophilic displacement in a curious way to produce α-oxoester.

 Please wait while we load your content...
Please wait while we load your content...