Elusive 2H-1,2-oxasiletes through reactions of an isolable dialkylsilylene with diazocarbonyl compounds†
Abstract
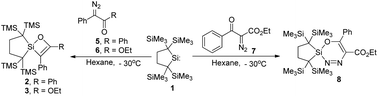
The reactions of isolable dialkysilylene 1 with 2-diazo-1,2-diphenylethanone and ethyl 2-diazo-2-phenylacetate gave elusive silacycles, 2H-1,2-oxasiletes 2 and 3, respectively, in high yields. Because these reactions occur at low temperatures of ca. −30 °C, initial complexation of the silylene to the carbonyl oxygen of the diazocarbonyl compounds is suggested to trigger dinitrogen elimination followed by cyclization. In contrast, a six-membered cyclic diazo compound 8 and 1-sila-2,3-diazabicyclo[3.3.0]oct-3-ene 10 were obtained in good yields by the reaction of 1 with less reactive ethyl 2-diazo-3-oxo-3-phenylpropanoate 7 and trimethylsilyldiazomethane 9. Molecular structures of 2, 3, 8 and 10 were determined by X-ray crystallography.

 Please wait while we load your content...
Please wait while we load your content...