Synthesis and biological evaluation of conformationally restricted adenine bicycloribonucleosides†
Abstract
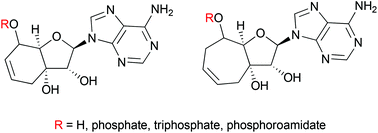
We prepared a novel series of conformationally restricted bicyclonucleosides and nucleotides. The synthetic approach employed a ring closing metathesis to provide access to both 6 and 7 membered saturated and unsaturated rings linking the 3′ to 5′ methylene groups of the sugar. The bicyclonucleosides were also transformed to the corresponding phosphoramidate prodrugs by an innovative one-pot protocol of boronate ester protection, coupling of the phosphoryl chloridate and deprotection of the boronate. A similar strategy was also employed for the synthesis of the corresponding monophosphates as crucial intermediates for the synthesis of selected triphosphates. The biological properties of the nucleosides and monophosphate prodrugs were assessed for antiviral and cytostatic activities in cell based assays whilst the triphosphates were evaluated in enzymatic assays. The lack of significant effects suggests that the linkage of the 3′ to 5′ via a ring system and the subsequent conformational restriction of the ribose ring to the South conformation are incompatible with the kinases and polymerases that recognize nucleosides and their metabolites.

 Please wait while we load your content...
Please wait while we load your content...