Copper-catalyzed efficient direct amidation of 2-methylquinolines with amines†
Abstract
A novel Cu-catalyzed direct amidation of 2-methylquinolines with amines is described. This method afforded an efficient approach for the synthesis of biologically important aromatic amides from readily available coupling partners using molecular oxygen as the oxidant.
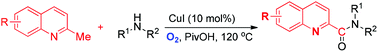

 Please wait while we load your content...
Please wait while we load your content...