Synthesis of tetraarylpyridines by chemo-selective Suzuki–Miyaura reactions of 3,5-dibromo-2,6-dichloropyridine†
Abstract
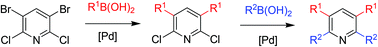
Chemoselective Suzuki–Miyaura reactions on 3,5-dibromo-2,6-dichloropyridine were studied. The optimized reaction conditions allow for the facile access of 3-aryl- and 3,5-diarylpyridines in good yields. Suzuki–Miyaura reactions of the selectively synthesized 2,6-dichloro-3,5-diarylpyridines gave the corresponding 2,3,5,6-tetraarylpyridines, containing two different aryl moieties.

 Please wait while we load your content...
Please wait while we load your content...