Synthesis of 8-aza-3,7-dideaza-2′-deoxyadenosines possessing a new adenosine skeleton as an environmentally sensitive fluorescent nucleoside for monitoring the DNA minor groove†
Abstract
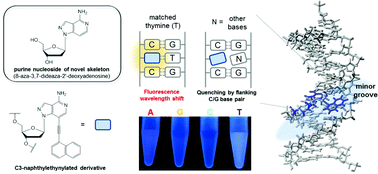
8-Aza-3,7-dideaza-2′-deoxyadenosine 1 and its C3-naphthylethynylated derivative 3n7zA (2) comprising a 8-aza-3,7-dideazapurine (pyrazolo[4,3-c]pyridine) skeleton were synthesized for the first time. In particular, nucleoside 3n7zA (2) exhibited environmentally sensitive intramolecular charge transfer (ICT) emission because of electron transition in the coplanar conformer formed by nucleobase and naphthalene moieties. Its incorporation into oligodeoxynucleotide (ODN) probes enable a clear identification of a perfectly matched thymine (T) in the complementary strand by a distinct change in the emission wavelength. In addition, the fluorescence emission of the duplexes containing a cytosine/guanine (C/G) base pair flanking 3n7zA (2) was strongly quenched by guanine only when the opposite base of the modified nucleoside was mismatched, enhancing its base identification ability. Thus, ODN probes containing 3n7zA (2) acted as effective reporter probes for homogeneous single nucleotide polymorphism (SNP) typing.

 Please wait while we load your content...
Please wait while we load your content...