Rapid and scalable synthesis of innovative unnatural α,β or γ-amino acids functionalized with tertiary amines on their side-chains†
Abstract
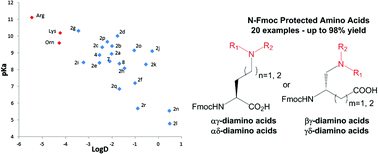
We report a selective ruthenium catalyzed reduction of tertiary amides on the side chain of Fmoc-Gln-OtBu derivatives, leading to innovative unnatural α,β or γ-amino acids functionalized with tertiary amines. Rapid and scalable, this process allowed us to build a library of basic unnatural amino acids at the gram-scale and directly usable for liquid- or solid-phase peptide synthesis. The diversity of available tertiary amines allows us to modulate the physicochemical properties of the resulting amino acids, such as basicity or hydrophobicity.

 Please wait while we load your content...
Please wait while we load your content...