Copper-catalysed ring-opening trifluoromethylation of cyclopropanols†
Abstract
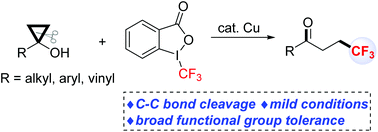
A copper-catalysed ring-opening trifluoromethylation reaction of cyclopropanols has been developed. Various β-trifluoromethyl ketones are obtained in good to excellent yields under mild reaction conditions. The present method also exhibits good functional-group compatibility. The mechanism of this new ring-opening trifluoromethylation reaction was investigated by radical trapping reactions.

 Please wait while we load your content...
Please wait while we load your content...