Phosphonate derivatives of tetraazamacrocycles as new inhibitors of protein tyrosine phosphatases†
Abstract
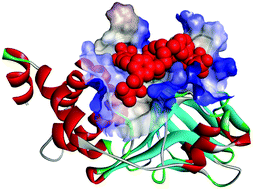
α,α-Difluoro-β-ketophosphonated derivatives of tetraazamacrocycles were synthesized and found to be potential inhibitors of protein tyrosine phosphatases. N-Substituted conjugates of cyclam and cyclen with bioisosteric phosphonate groups displayed good activities toward T-cell protein tyrosine phosphatase with IC50 values in the micromolar to nanomolar range and showed selectivity over PTP1B, CD45, SHP2, and PTPβ. Kinetic studies indicated that the inhibitors can occupy the region of the active site of TC-PTP. This study demonstrates a new approach which employs tetraazamacrocycles as a molecular platform for designing inhibitors of protein tyrosine phosphatases.

 Please wait while we load your content...
Please wait while we load your content...