Titanium carbenoid-mediated cyclopropanation of allylic alcohols: selectivity and mechanism†
Abstract
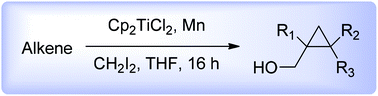
A new method for the chemo- and stereoselective conversion of allylic alcohols into the corresponding cyclopropane derivatives has been developed. The cyclopropanation reaction was carried out with an unprecedented titanium carbenoid generated in situ from Nugent's reagent, manganese and methylene diiodide. The reaction involving the participation of an allylic hydroxyl group, proceeded with conservation of the alkene geometry and in a high diastereomeric excess. The scope, limitations and mechanism of this metal-catalysed reaction are discussed.


 Please wait while we load your content...
Please wait while we load your content...