Thermodynamics of various F420 coenzyme models as sources of electrons, hydride ions, hydrogen atoms and protons in acetonitrile†
Abstract
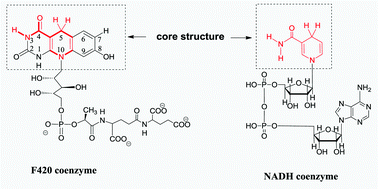
32 F420 coenzyme models with alkylation of the three different N atoms (N1, N3 and N10) in the core structure (XFH−) were designed and synthesized and the thermodynamic driving forces (defined in terms of the molar enthalpy changes or the standard redox potentials in this work) of the 32 XFH− releasing hydride ions, hydrogen atoms and electrons, the thermodynamic driving forces of the 32 XFH˙ releasing protons and hydrogen atoms and the thermodynamic driving forces of XF−˙ releasing electrons in acetonitrile were determined using titration calorimetry and electrochemical methods. The effects of the methyl group at N1, N3 and N10 and a negative charge on N1 and N10 atoms on the six thermodynamic driving forces of the F420 coenzyme models and their related reaction intermediates were examined; the results show that seating arrangements of the methyl group and the negative charge have remarkably different effects on the thermodynamic properties of the F420 coenzyme models and their related reaction intermediates. The effects of the substituents at C7 and C8 on the six thermodynamic driving forces of the F420 coenzyme models and their related reaction intermediates were also examined; the results show that the substituents at C7 and C8 have good Hammett linear free energy relationships with the six thermodynamic parameters. Meanwhile, a reasonable determination of possible reactions between members of the F420 family and NADH family in vivo was given according to a thermodynamic analysis platform constructed using the elementary step thermodynamic parameter of F420 coenzyme model 2FH− and NADH model MNAH releasing hydride ions in acetonitrile. The information disclosed in this work can not only fill a gap in the chemical thermodynamics of F420 coenzyme models as a class of very important organic sources of electrons, hydride ions, hydrogen atoms and protons, but also strongly promote the fast development of the chemistry and applications of F420 coenzyme.

 Please wait while we load your content...
Please wait while we load your content...