Effect of preorganization on the affinity of synthetic DNA binding motifs for nucleotide ligands†
Abstract
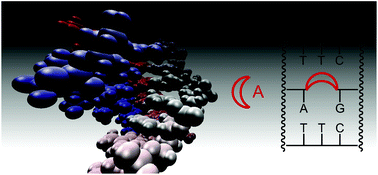
Triplexes with a gap in the purine strand have been shown to bind adenosine or guanosine derivatives through a combination of Watson–Crick and Hoogsteen base pairing. Rigidifying the binding site should be advantageous for affinity. Here we report that clamps delimiting the binding site have a modest effect on affinity, while bridging the gap of the purine strand can strongly increase affinity for ATP, cAMP, and FAD. The lowest dissociation constants were measured for two-strand triple helical motifs with a propylene bridge or an abasic nucleoside analog, with Kd values as low as 30 nM for cAMP in the latter case. Taken together, our data suggest that improving preorganization through covalent bridges increases the affinity for nucleotide ligands. But, a bulky bridge may also block one of two alternative binding modes for the adenine base. The results may help to design new receptors, switches, or storage motifs for purine-containing ligands.

 Please wait while we load your content...
Please wait while we load your content...