Triazolophostins: a library of novel and potent agonists of IP3 receptors†
Abstract
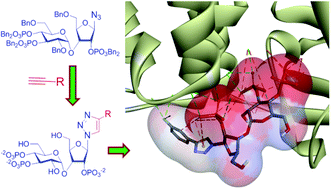
IP3 receptors are channels that mediate the release of Ca2+ from the intracellular stores of cells stimulated by hormones or neurotransmitters. Adenophostin A (AdA) is the most potent agonist of IP3 receptors, with the β-anomeric adenine contributing to the increased potency. The potency of AdA and its stability towards the enzymes that degrade IP3 have aroused interest in AdA analogs for biological studies. The complex structure of AdA poses problems that have necessitated optimization of synthetic conditions for each analog. Such lengthy one-at-a-time syntheses limit access to AdA analogs. We have addressed this problem by synthesizing a library of triazole-based AdA analogs, triazolophostins, by employing click chemistry. An advanced intermediate having all the necessary phosphates and a β-azide at the anomeric position was reacted with various alkynes under Cu(I) catalysis to yield triazoles, which upon deprotection gave triazolophostins. All eleven triazolophostins synthesized are more potent than IP3 and some are equipotent with AdA in functional analyses of IP3 receptors. We show that a triazole ring can replace adenine without compromising the potency of AdA and provide facile routes to novel AdA analogs.

 Please wait while we load your content...
Please wait while we load your content...