Rational design, synthesis and molecular modeling studies of novel anti-oncological alkaloids against melanoma†
Abstract
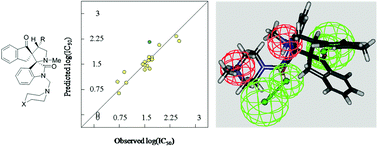
3D-pharmacophore and 2D-QSAR modeling studies describe the anti-oncological properties of spiro-alkaloids. The dispiro[2H-indene-2,3′-pyrrolidine-2′,3′′-[3H]indole]-1,2′′(1′′H, 3H)-diones 20–38 were prepared via 1,3-dipolar cycloaddition reactions of azomethine ylides (generated in situ via decarboxylative condensation of isatins 7–9 with sarcosine 10) and 2-(arylmethylidene)-2,3-dihydro-1H-inden-1-ones 11–19 in refluxing ethanol. Some of the spiro-alkaloids (21, 22, 29 and 37) revealed potent antitumor properties against melanoma carcinoma cell lines (GaLa, LuPiCi and LuCa) utilizing the in vitro SRB standard method exhibiting potency close to that of the standard reference doxorubicin.

 Please wait while we load your content...
Please wait while we load your content...