Resveratrol-based benzoselenophenes with an enhanced antioxidant and chain breaking capacity†
Abstract
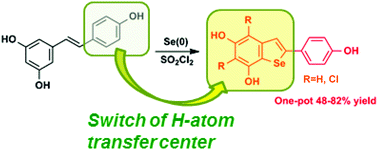
The structural modification of the resveratrol scaffold is currently an active issue in the quest for more potent and versatile antioxidant derivatives for biomedical applications. Disclosed herein is an expedient and efficient entry to a novel class of resveratrol derivatives featuring an unprecedented 2-phenylbenzoselenophene skeleton. The new compounds were obtained in good yields by direct selenenylation of resveratrol with Se(0) and SO2Cl2 in dry THF. Varying the [Se : SO2Cl2 : resveratrol] ratio resulted in the formation of the parent benzoselenophene (1) and/or mono (2) and/or dichloro (3) benzoselenophene derivatives. All the benzoselenophene derivatives proved to be more efficient than resveratrol in the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ferric reducing/antioxidant power (FRAP) assays, with 1 showing an activity nearly comparable to that of Trolox. 1–3 also proved to be more efficient inhibitors than the parent resveratrol in kinetic experiments of styrene autoxidation. DFT calculations of the O–H bond dissociation enthalpy (BDE) revealed that the introduction of the Se-atom causes a significant decrease of the BDE of 3-OH and 5-OH, with just a small increase of the 4′-OH BDE. Compounds 1–3 showed no cytotoxicity at 5 μM concentrations on human keratinocyte (HaCaT) and intestinal (CaCo-2) cell lines.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST

 Please wait while we load your content...
Please wait while we load your content...