Synthesis and structural reconfirmation of bacillamide B†
Abstract
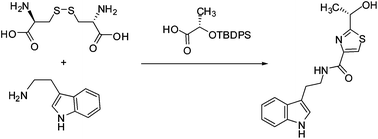
A concise total synthesis of the natural product bacillamide B was accomplished in 30% overall yield starting from L-cystine. The key step was a one-pot four-step process of thiazoline formation via a cascade disulfide cleavage/thiocarbonylation/Staudinger reduction/aza-Wittig reaction using β-azido disulfide and carboxylic acid as substrates. The absolute configuration of the natural bacillamide B was reconfirmed to be S and the specific optical rotation was revised to (−).

 Please wait while we load your content...
Please wait while we load your content...