Palladium-catalyzed regio-selective oxidative C–H bond acylation of azoxybenzenes with alcohols†
Abstract
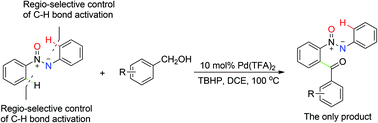
A palladium-catalyzed regio-selective acylation of C–H bonds of azoxybenzenes with alcohols was developed using tert-butyl hydroperoxide (TBHP) as an oxidant. Alcohol derivatives can act as effective acyl precursors in situ, which are less toxic, inexpensive, stable, and commercially available. These transformations proceeded smoothly and could tolerate a variety of functional groups.

 Please wait while we load your content...
Please wait while we load your content...