Enantioselective 6-endo bromoaminocyclization of 2,4-dienyl N-tosylcarbamates catalyzed by a chiral phosphine oxide-Sc(OTf)3 complex. A dramatic additive effect†
Abstract
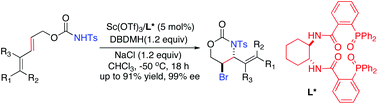
An effective enantioselective 6-endo bromoaminocyclization of 2,4-dienyl N-tosylcarbamates catalyzed by a chiral phosphine oxide-Sc(OTf)3 complex is described. A wide variety of optically active 5-bromo-1,3-oxazinan-2-ones containing various functional groups can be obtained in 61–91% yields and 92–99% ees. An additive, such as NaCl, has been found to be crucial for the reaction process.

 Please wait while we load your content...
Please wait while we load your content...