A novel enantioselective synthesis of 6H-dibenzopyran derivatives by combined palladium/norbornene and cinchona alkaloid catalysis†
Abstract
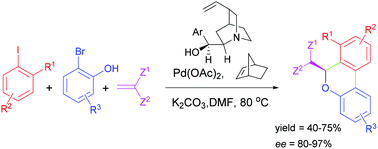
Organometallic and organo-catalysts are cooperatively at work in the enantioselective synthesis of dibenzopyran derivatives; palladium/norbornene and a cinchona alkaloid base guarantee good yields and satisfactory enantioselectivities in a one-pot reaction.

 Please wait while we load your content...
Please wait while we load your content...