Alkylidene malonates and α,β-unsaturated α′-hydroxyketones as practical substrates for vinylogous Friedel–Crafts alkylations in water catalysed by scandium(iii) triflate/SDS†
Abstract
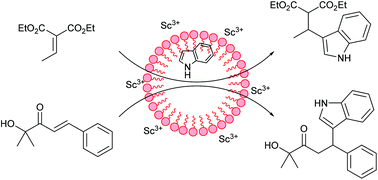
Alkylidene malonates and α,β-unsaturated α′-hydroxyketones are demonstrated to be efficient classes of electrophiles for the scandium(III) triflate/sodium dodecyl sulphate (SDS) catalysed vinylogous Friedel–Crafts alkylation of indoles and pyrroles in water. These substrates contain an easily removable auxiliary group that increases affinity for the catalytic metal ion in such a way that they can compete with water for binding to the catalytic metal ion. Thus, alkylidene malonates and α,β-unsaturated α′-hydroxyketones are attractive substitutes for, e.g., α,β-unsaturated carboxylic acids and -esters, which in aqueous media are not reactive enough in these reactions. The combination of Lewis acid and SDS in catalysis results in considerable acceleration of the reaction in water compared to organic solvents. The method presented is attractive because the reactions are fast, experimentally straightforward and give rise to high yields of products.

 Please wait while we load your content...
Please wait while we load your content...