“One-pot” synthesis of amidoxime via Pd-catalyzed cyanation and amidoximation†
Abstract
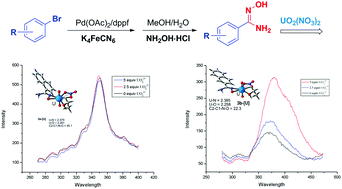
A novel “one-pot” reaction was developed for the synthesis of aryl or heteroaryl-substituted amidoxime compounds containing various functional groups. Fluorescence titration experiments coupled with theoretical analysis revealed that the steric hindrance and electronic effects of substituents influence the binding ability of the amidoxime compounds to uranyl ions.

 Please wait while we load your content...
Please wait while we load your content...