Studies towards asymmetric synthesis of 4(S)-11-dihydroxydocosahexaenoic acid (diHDHA) featuring cross-coupling of chiral stannane under mild conditions†
Abstract
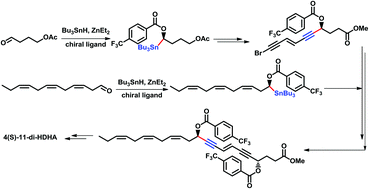
An efficient and asymmetric synthetic approach towards one of the biologically interesting 4(S)-11-diHDHA derivatives was developed. This process mainly relied on two reactions, one is the copper-catalyzed mild cross-coupling that allows for the efficient construction of a chiral α-alkynyl α-hydroxy motif and another is the synthesis of chiral α-hydroxy α-stannanes that has previously been developed by our group featuring the asymmetric stannylation using the well-established tributyltin hydride/diethyl zinc system from an aldehyde.

 Please wait while we load your content...
Please wait while we load your content...