Developing piperine towards TRPV1 and GABAA receptor ligands – synthesis of piperine analogs via Heck-coupling of conjugated dienes†
Abstract
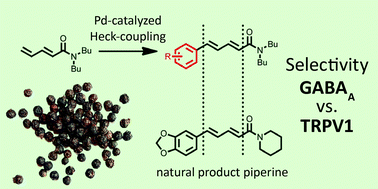
Piperine, the pungent alkaloid of black pepper, and several of its derivatives are modulators of γ-amino butyric acid type A (GABAA) receptors. Concomitantly, this natural product has also been reported to activate transient receptor potential vanilloid type 1 (TRPV1) receptors. We have developed a Heck cross-coupling reaction of conjugated dienamides enabling the rapid assembly of piperine derivatives containing a modified aromatic core. Upon assessment of a focussed compound library, key aromatic substituents were identified selectively affecting either the GABAA or the TRPV1 receptor.

 Please wait while we load your content...
Please wait while we load your content...