Syntheses of arabinose-derived pyrrolidine catalysts and their applications in intramolecular Diels–Alder reactions†
Abstract
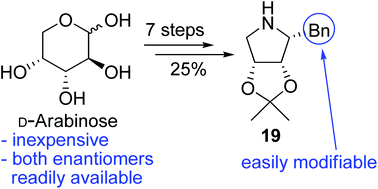
Six chiral hydroxylated pyrrolidine catalysts were synthesized from commercially available D-arabinose in seven steps. Various aromatic substituents α to the amine can be introduced readily by a Grignard reaction, which enables facile optimization of the catalyst performance. The stereoselectivities of these catalysts have been assessed by comparing with those of MacMillan's imidazolidinone in a known intramolecular Diels–Alder (IMDA) reaction of a triene. Two additional IMDA reactions of symmetrical dienals with concomitant desymmetrisation further established the potential use of these novel amine catalysts. These pyrrolidines are valuable catalysts for other synthetic transformations.

 Please wait while we load your content...
Please wait while we load your content...