pH response and molecular recognition in a low molecular weight peptide hydrogel†
Abstract
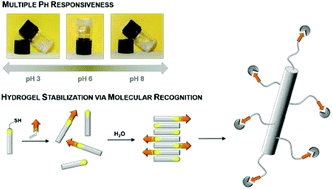
In this article we report the preparation and characterization of a peptide-based hydrogel, which possesses characteristic rheological properties, is pH responsive and can be functionalized at its thiol function. The tripeptide N-(fluorenyl-9-methoxycarbonyl)-L-Cys(acetamidomethyl)-L-His-L-Cys-OH 1 forms stable supramolecular aggregates in water leading to hydrogels above 1.5 wt%. Rheological analysis of the hydrogel revealed visco-elastic and shear thinning properties of samples containing 1.5 wt% of peptide 1. The hydrogel reversibly responds to pH changes. Below and above pH 6, electrostatic repulsion of the peptide results in a weakening of the three-dimensional gel network. Based on atomic force microscopy, small angle X-ray scattering and molecular dynamics simulations, it is proposed that the peptide assembles into nanostructures that tend to entangle at higher concentrations in water. The development of functional materials based on the peptide assemblies was possible via thiol–ene-click chemistry of the free thiol function at the C-terminal cysteine unit. As a proof of concept, the functionalization with adamantyl units to give 1-Ad was shown by molecular recognition of β-cyclodextrin vesicles. These vesicles were used as supramolecular cross-linkers of the assemblies of peptide 1 mixed with peptide 1-Ad leading to gel networks at a reduced peptide concentration.
- This article is part of the themed collection: Supramolecular Chemistry in Water

 Please wait while we load your content...
Please wait while we load your content...