Enabling the (3 + 2) cycloaddition reaction in assembling newer anti-tubercular lead acting through the inhibition of the gyrase ATPase domain: lead optimization and structure activity profiling†
Abstract
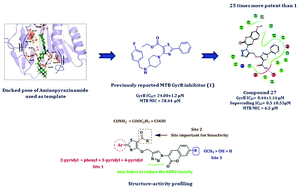
DNA gyrase, the sole type II topoisomerase present in Mycobacterium tuberculosis, is absent in humans and is a well validated target for anti-tubercular drug discovery. In this study, a moderately active inhibitor of Mycobacterium tuberculosis GyrB, the pharmaceutically unexploited domain of DNA gyrase, was reengineered using a combination of molecular docking and medicinal chemistry strategies to obtain a lead series displaying considerable in vitro enzyme efficacy and bacterial kill against the Mycobacterium tuberculosis H37Rv strain. Biophysical investigations using differential scanning fluorimetry experiments re-ascertained the affinity of these molecules towards the GyrB domain. Furthermore, the molecules were completely devoid of hERG toxicity up to 30 μM, as evaluated in a zebra fish model with a good selectivity index, and from a pharmaceutical point of view, turned out as potential candidates against TB.

 Please wait while we load your content...
Please wait while we load your content...