Synthesis and evaluation of protein arginine N-methyltransferase inhibitors designed to simultaneously occupy both substrate binding sites†
Abstract
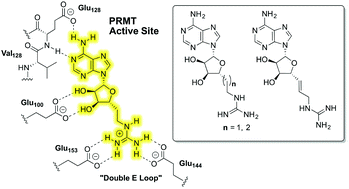
The protein arginine N-methyltransferases (PRMTs) are a family of enzymes that function by specifically transferring a methyl group from the cofactor S-adenosyl-L-methionine (AdoMet) to the guanidine group of arginine residues in target proteins. The most notable is the PRMT-mediated methylation of arginine residues that are present in histone proteins which can lead to chromatin remodelling and influence gene transcription. A growing body of evidence now implicates dysregulated PRMT activity in a number of diseases including various forms of cancer. The development of PRMT inhibitors may therefore hold potential as a means of developing new therapeutics. We here report the synthesis and evaluation of a series of small molecule PRMT inhibitors designed to simultaneously occupy the binding sites of both the guanidino substrate and AdoMet cofactor. Potent inhibition and surprising selectivity were observed when testing these compounds against a panel of methyltransferases.

 Please wait while we load your content...
Please wait while we load your content...