Synthesis of substituted azafluorenones from dihalogeno diaryl ketones by palladium-catalyzed auto-tandem processes†
Abstract
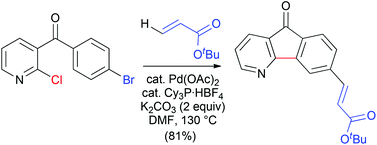
Substituted azafluorenones were synthesized from different dihalogeno diaryl ketones under palladium catalysis by combining either Suzuki or Heck coupling with direct cyclizing arylation. Conditions were identified to allow both auto-tandem processes to proceed successfully from 3-(bromobenzoyl)- or 3-benzoyl-4-bromo-2-chloropyridines, as well as 4-benzoyl-2,3- and 4-benzoyl-2,5-dichloropyridines.

 Please wait while we load your content...
Please wait while we load your content...