Synthesis of substituted quinolines via allylic amination and intramolecular Heck-coupling†
Abstract
A new catalytic approach for the synthesis of substituted quinolines via C–N and C–C bond formation using 2-haloaryl hydroxylamines and allylic C–H substrates is described. Fe-catalyzed allylic C–H amination followed by Pd-catalyzed intramolecular Heck-coupling and aerobic dehydrogenation deliver the valuable quinoline and naphthyridine heterocycles in good to excellent overall yields. In this process, Pd(OAc)2 plays a dual role in catalyzing Heck coupling as well as aerobic dehydrogenation of dihydroquinolines.
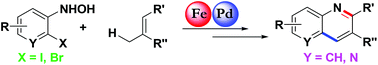


 Please wait while we load your content...
Please wait while we load your content...