A review of methods to synthesise 4′-substituted nucleosides
Abstract
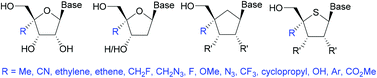
Modified nucleosides have received a great deal of attention from the scientific community, either for use as therapeutic agents, diagnostic tools, or as molecular probes. Perhaps the most difficult position of a nucleoside to modify is the 4′-position. Chemists have developed innovative methods to achieve this in a stereoselective manner to allow incorporation of a variety of functional groups. This review provides a summary of the most commonly used or recently published methods for ribose, deoxy-ribose, 4′-thioribose, and carbocyclics.

 Please wait while we load your content...
Please wait while we load your content...