The importance of chain conformational mobility during 5-exo-cyclizations of C-, N- and O-centred radicals†
Abstract
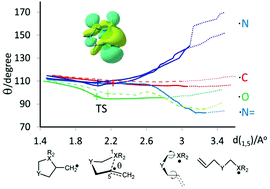
The reaction coordinates of an archetypical set of 5-exo cyclizations of C-, N- and O-centred radicals were investigated by computational methods. G4 theory, and DFT with the um062x functional, were able to rationalise counterintuitive factors such as the ‘normal’ order of rate constants being: N-centred < C-centred < O-centred radicals. The access angle between the radical centre and the double bond was identified as a key factor. Examination of its evolution during ring closure implied that rigidity at the N-ends of the chains, and the consequent extra energy needed to attain chair-like transition states, might be the reason for slow aminyl cyclizations. A novel linear correlation between cyclization activation energies and the access angles was discovered. The preference for cis-1,2-disubstituted product formation was also accounted for in terms of interaction between the hyperconjugatively delocalized SOMO and the alkene π* orbital.

 Please wait while we load your content...
Please wait while we load your content...