Silver triflate and triflic anhydride-promoted expedient synthesis of acylated 1-aminoisoquinolines†
Abstract
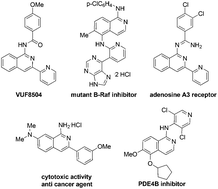
A practical and convergent synthesis of biologically active 1-(N-acyl)-1-aminoisoquinolines from the reaction of 2-alkynylbenzaldoximes with amides has been realized. The readily available amides could be activated with triflic anhydride (Tf2O) and could efficiently participate in the domino reaction of 2-alkynylbenzaldoximes when catalyzed by AgOTf, thus providing various acylated 1-aminoisoquinolines with up to 98% yields.

 Please wait while we load your content...
Please wait while we load your content...